YONDELIS® is the first FDA-approved treatment for liposarcoma and leiomyosarcoma studied against an active comparator in a phase 3 trial.1
VIEW THE STUDY DESIGN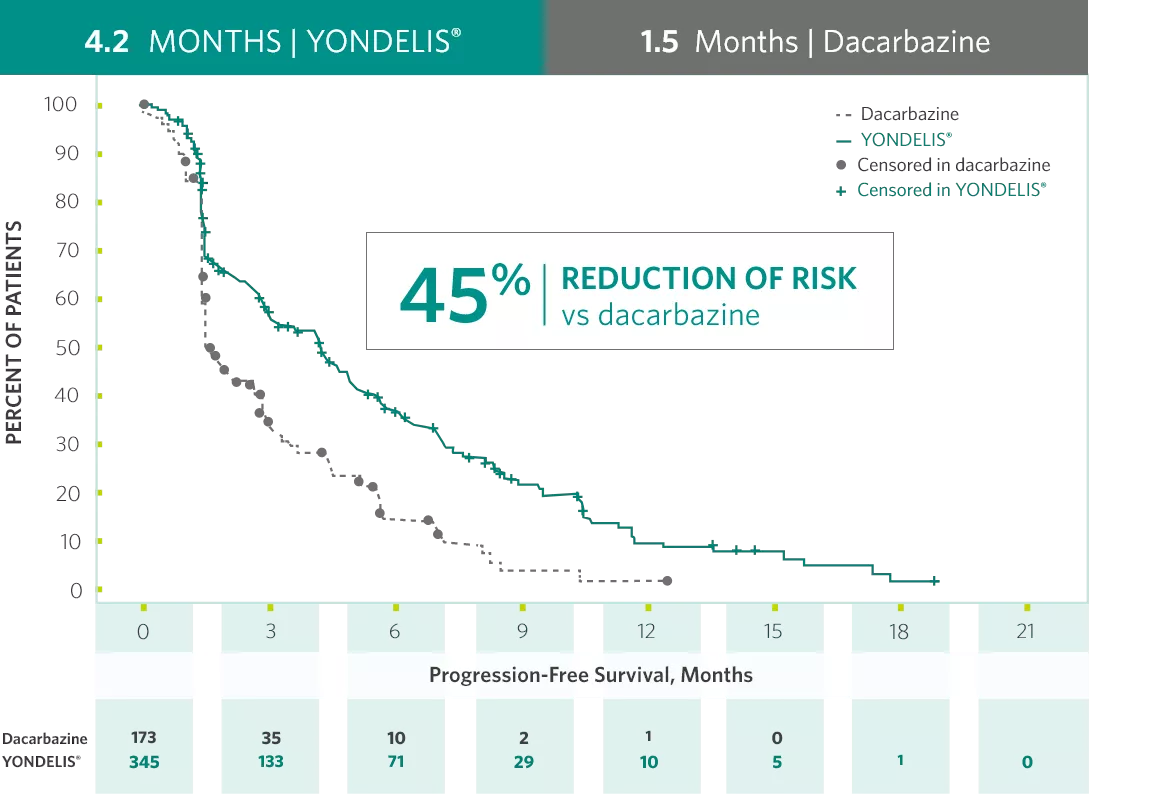
(HR†=0.55; 95% CI: 0.44, 0.70; P<0.001‡)1
An exploratory analysis of independent radiology committee-determined PFS,§ in a subgroup consisting of approximately 60% of the total population, provided similar results to the investigator-determined PFS.
Hazard ratio (HR) is estimated using Cox proportional hazards model with treatment group as the only covariate.
P value is based on unstratified log-rank test.
The time from randomization to the occurrence of disease progression or death, whichever occurred first.2
CI=confidence interval; FDA=US Food and Drug Administration; HR=hazard ratio.
Overall Survivalll (median)1:
(HR†=0.93; 95% CI: 0.75, 1.15; P=0.49‡)
Objective Response Rate (CR+PR)1:
(95% CI¶: 4.3, 9.8)
Duration of Response (CR+PR) (median)1:
(95% CI: 4.5, 7.6)
Overall survival=the time between randomization and death from any cause.2
Objective response rate=percentage of patients achieving complete response or partial response.2
Duration of response=duration of response for patients with complete response or partial response.2
CR=complete response; NE=not estimable; PR=partial response.
HR is estimated using Cox proportional hazards model with treatment group as the only covariate.1
P value is based on unstratified log-rank test.1
Based on 384 patients randomized to the YONDELIS® arm and 193 patients randomized to dacarbazine.1
Fisher’s exact CI.1
Continued on YONDELIS® treatment for 6 cycles or more3
Continued on YONDELIS® treatment for 12 cycles or more3
YONDELIS® was studied in a phase 3 randomized, open-label, active-controlled, multicenter trial of patients (N=518) with unresectable, locally advanced or metastatic leiomyosarcomas (73%) or liposarcomas (27%).
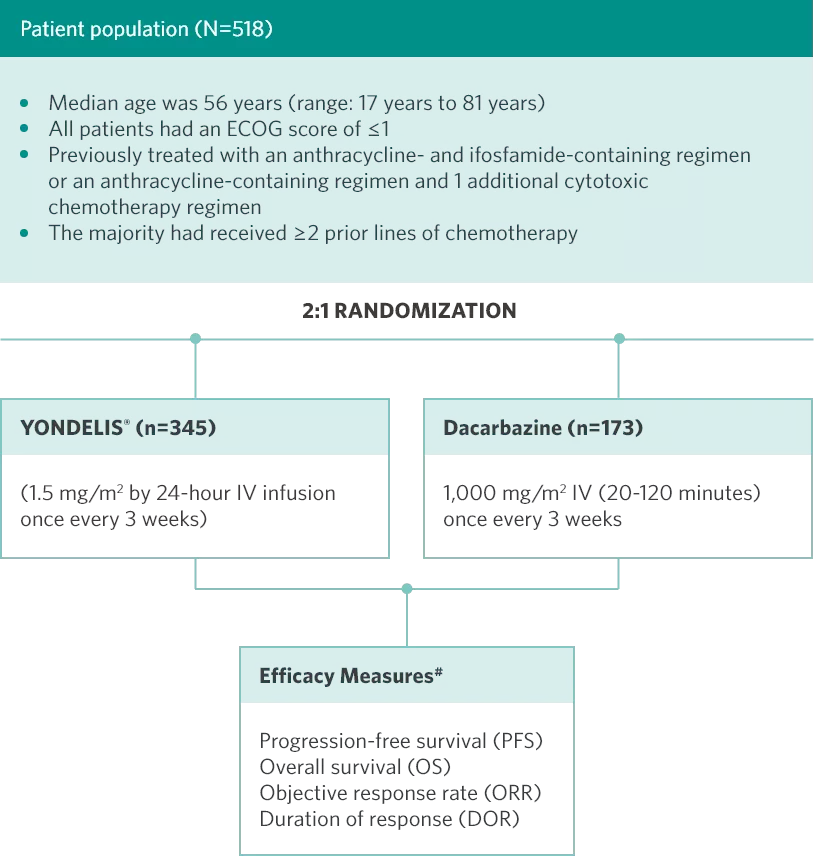
#Investigator-assessed.
ECOG=Eastern Cooperative Oncology Group; IV=intravenous.
See the safety results in clinical trials.
REFERENCES: